Daily Update 3.4
Week 5: Module 3: Date 3/1/31
Dissolved Oxygen (DO) is exactly what it sounds like. It is a measure of the dissolved oxygen in a medium.
The amount of dissolved oxygen that the water can hold depends on the temperature and salinity of the water. Cold water can hold more dissolved oxygen than warm water and fresh water can hold more dissolved oxygen than salt water. So the warmer and saltier the water, the less dissolved oxygen there can be. Oxygen enters the water at the surface of the water where exchange between the atmosphere and the water can take place. Waves and wind help put oxygen into the water.*
The other way oxygen enters the ocean is through photosynthesis of aquatic plants.
Just like on the land, there are also photosynthetically active plants and bacteria in the ocean, the primary producers. Annually, they generate about the same amount of oxygen and fix as much carbon as all the land plants together. This is quite amazing. After all, the total living biomass in the ocean is only about one two-hundredth of that in the land plants. This means that primary producers in the ocean are around two hundred times more productive than land plants with respect to their mass.**
This image shows how DO cycles through the ocean.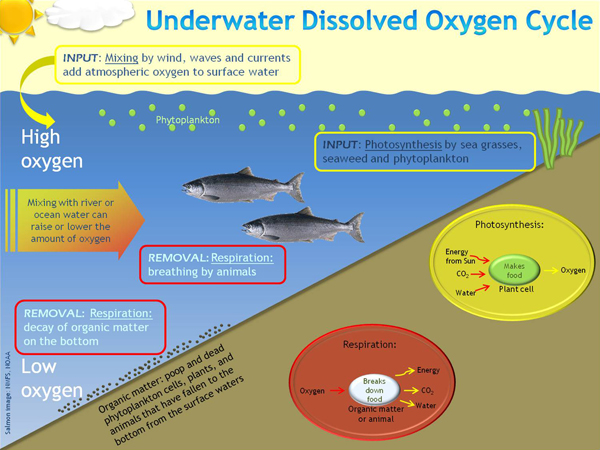
Oxygen in the ocean is something that has been a hot topic of late. This is because of the expansion of the dead zones that are occurring. A dead zone is an area in which the oxygen levels become too low to sustain life. At this point there are over 400 dead zones in the world’s oceans. This map shows where they are occurring. What do you notice about the location of the dead zones?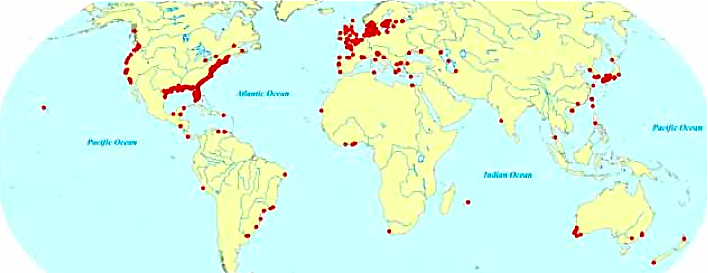
Almost all of these dead zones occur near highly developed coastlines. Why is that? This image shows how these dead zone form.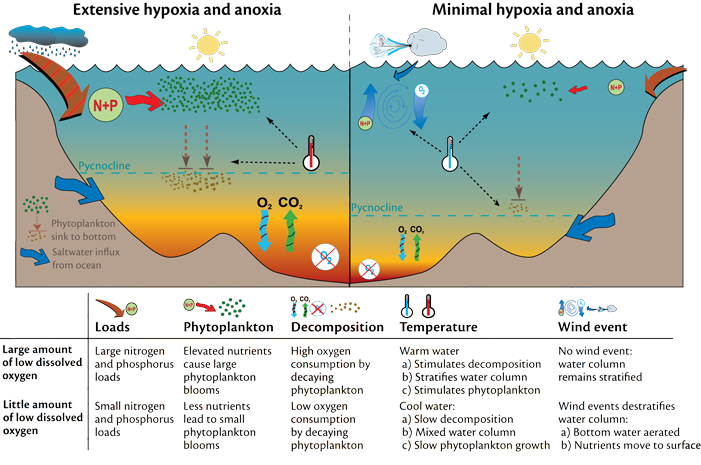
The increase in runoff that is high in phosphorus and nitrogen can cause phytoplankton blooms that can be seen from space like this one. Although the blooms do happen naturally as well, usually as a result of an increase in nutrients due to an upwelling that carries nutrient rich water up from the bottom of the ocean.
It is important to note that these dead zones do occur naturally as well but often are much smaller in size. DO is one of the measurements that can be used to quickly determine the ‘health’ of the ocean. It is also being monitored in relation to climate change as some models predict a decrease in DO in the ocean. Dissolved oxygen is another of the scientific measurements the crew of the JRH are collecting on their journey.
Sources and more information:
* http://omp.gso.uri.edu/ompweb/doee/science/physical/choxy1.htm
** http://worldoceanreview.com/en/ocean-chemistry/oxygen/
http://oceanservice.noaa.gov/education/kits/estuaries/media/supp_estuar10d_disolvedox.html

