Daily Update
Week 2: Module 1: 2/5/13
Water, water everywhere but not a drop to drink – Samuel Coleridge
This quote seems to be everyone’s favorite during an ocean trip, but due to the advancement of portable desalination units we can now turn salt water into potable (drinkable) water. In the past, water was one of the biggest limiting factors in open ocean travel and it was some of the most precious cargo.
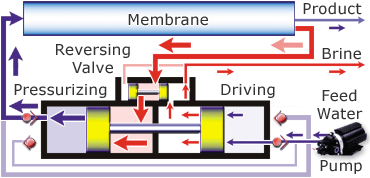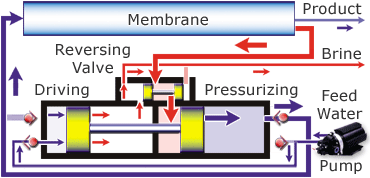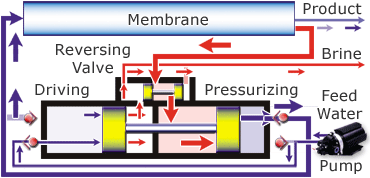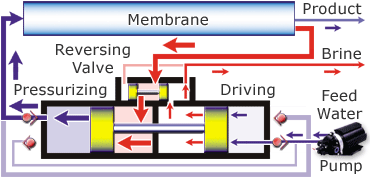
Desalination is possible in a variety of ways. You can use distillation, which uses heat to evaporate the water leaving the salt and other minerals behind and then you recapture the pure H2O as condensation. This isn’t a practical way because of the huge amount of fuel needed to evaporate the water. Another way to desalinate the water is through a process called reverse osmosis. In this process the saltwater is passed through a membrane and comes out as pure water. This isn’t just any membrane, it takes a special one and quite a bit of pressure to force the water through it. There are also additional pre-filters to remove the larger particles.
The diagram below shows how the Spectra Ventura 150, the model that is on the JRH, works.
This all comes at a cost. While it is more efficient than burning fuel to evaporate water, the power usage of a desalination unit is something that has to be carefully considered. The unit aboard the JRH uses about 102 watts to purify 6 gallons of water in one hour. In comparison an average laptop uses about 30 watts for one hour. This requires quite a bit of deliberation from the crew, first of all in deciding how many batteries to bring and also what instruments you can run during the day and still have water to drink. Not a calculation with a lot of room for error!
If you had to choose between charging your iphone or drinking water today which would it be?